Contents
- 1 Essential properties required in a refrigerant are as follows:
- 2 High latent heat of vaporization
- 3 A High Suction gas density and a Low Compression ratio.
- 4 Non-corrosive, non-toxic and non-flammable
- 5 High critical temperature
- 6 Compatibility with the lubricating oil
- 7 What is High latent heat
- 8 Low boiling point of refrigerant
- 9 Low condensing pressure
- 10 High dielectric strength (for compressors with integral motors)
- 11 It should be cheap and readily available
- 12 Ease of leak detection
- 13 Refrigerant gas used in World War
- 14 What are Environment-friendly Green Refrigerants?
Essential properties required in a refrigerant are as follows:

High latent heat of vaporization
High latent heat of vaporization is desirable in a refrigerant. It results in a high refrigerating effect and a low rate of refrigerant in circulation.
More heat can be absorbed and ejected in each cycle of refrigeration.
A High Suction gas density and a Low Compression ratio.
High suction gas density at the compressor suction means low sized compressor and a lesser-powered compressor motor. After the refrigerant passes through the evaporator, the refrigerant gas expansion is not very high, ie. Fluid to Gas expansion is low.
This property of refrigerant provided a low compression ratio for the compressor, the higher mass flow rate of the refrigerant leading to a low rate of circulation within the system.
Non-corrosive, non-toxic and non-flammable
Refrigerant must not be poisonous or lethal to air-conditioning, human health, and foodstuff. When coming in contact with metal parts like piping and compressor must not cause any sort of electrochemical corrosion.
Must be compatible with non-metallic parts such as nitrile rubber tubes, O-rings (used for sealing purpose).
As the refrigerant gets compressed under high pressure and temperature inside the compressor, it must not cause any explosion or catch fire within the system and continue its non-flammable properties.
The presence of moisture in the system may cause the formation of highly corrosive compounds (usual acids) which may react with compressor lubricating oil and with other materials in the system including metals.
Moisture may cause the failure of compressor valves in case of hermetic compressor often causes breakdown of the motor winding insulation resulting in short-circuiting or grounding of the motor.
The presence of moisture in lubricating oil may deteriorate properties of lubricating oil and formation of metallic or other sludge which may lead to clogging or chocking of valves, filters, and other oil passages.
Moisture can enter into the system while charging of refrigerant, while repair ie. evacuation or vacuuming of the system where moisture can enter through leaky joints, moisture can exist as free water although, a completely moisture free refrigerating system is not possible.
When moisture is present in the form of water it may lead to icing within the evaporator coil and choking of the thermostatic expansion valve.
Avoid moisture formation by connecting a filter drier which absorbs moisture from the refrigerant.
High critical temperature
It is the temperature above which the vapour refrigerant remains in the vapour state and cannot be liquefied back into its liquid state even after passing through the condenser or any cooling medium at any given pressure.
This happens only when the refrigerant temperature reaches beyond its critical temperature, ie. when a refrigerant has a low critical temperature.
So, it’s better to select a refrigerant having a high critical temperature, else the vapour refrigerant after getting compressed inside the compressor into a hot vapour will not be condensed into a liquid after passing inside condenser coil.
To be able to condense high-temperature vapour refrigerant into a liquid refrigerant by passing outside hot air in summer via a fan over condenser coils, the refrigerant must have high latent heat of vaporization.
Compatibility with the lubricating oil
The refrigerant used must be compatible and miscible (means refrigerant can be easily separated) with the compressor oil as there are greater chances of intermixing inside the compressor, wherein such condition following problems can occur:
- Acid or sludge formation.
- Acidic corrosion decreases alkalinity in oil.
- The decrease in viscosity leading to insufficient lubrication.
- Oil carbonization with the rise in temperature.
- Compressor damage due to the loss of the lubricating oil properties and load carrying property.
What is High latent heat
Latent heat means the amount of heat required by the refrigerant to change its state from liquid to vapour.
With a high latent heat, the refrigerant absorbs more heat from the load; this increases the system refrigeration efficiency. Also, it reduces the required mass flow rate and the refrigerant quantity.
Low boiling point of refrigerant
The low boiling point allows the refrigerant to boil off into vapor at a lower temperature. The refrigerant enters the evaporator coils in liquid state and leaves as vapour.
As the refrigerant leaves the evaporator, the refrigerant has to be in 100 % vapour form, to avoid ingress of liquid refrigerant inside compressor as the liquid is incompressible.
Also, with low boiling point refrigerant property, the low-temperature refrigerant can easily absorb the room heat and convert into vapour form.
Therefore with a low boiling point, we can maintain a low temperature like at home air-conditioner we can set the temperature up to +16°C whereas for household refrigerator we maintain lower temperatures of -5°C and for the commercial system we can maintain as low as -25°C.
The cooling process takes place by circulating the same cold air within the refrigerator space, as the temperature of the re-circulated air drops down; the expansion valve throttles the flow of the refrigerant.
Now, the point here is the re-circulated cold air when passes through the evaporator; the refrigerant is able to extract the latent heat from the cold air to convert to 100% vapor due to the property of low boiling point.
Low condensing pressure
Lower condenser pressure reduces the power drawn by the compressor during compression thereby giving a smaller sized compressor.
The condenser tubes don’t have to handle high-pressure refrigerants; which reduces the overall design cost.
High dielectric strength (for compressors with integral motors)
Refrigerant with a high dielectric strength avoids short circuits when refrigerant comes directly in contact with motor windings in a hermetically sealed compressor.
The dielectric strength of any material is a measure of its insulation property.
It should be cheap and readily available
In case of a repair, maintenance or accidental leakage; refrigerant must be readily available at a reasonable price.
Ease of leak detection
Leaks can be detected by smell, using soap solution over the connections, using pressure testing method by keeping the line under pressure for 20 to 30 minutes to check for a drop in pressure.
U.V leak detection method where we inject a small amount of fluorescent dye into the running refrigeration system and later scan the system with a leak detection lamp. The dye escapes from the leaky sites and shows up as a green or yellow solution.
Perform a halide leak test detection on a refrigerant that are Halogenated Hydrocarbons (Freon compounds). It involves holding a torch or a flame close to the leak area. If there is a refrigerant leak, the flame goes green. In every instance, the halide torch test are carried out in a well-ventilated room.
An electronic detector for such refrigerant leaks is presently available. The detector gives off a chain of rapid clicks if the refrigerant is present. The elevated the concentration of the refrigerant, the more rapid the clicks.
Refrigerant gas used in World War
In certain concentrations and the presence of an open flame such as a gas range or a gas water heater, R-12 and R-22 may break down and form a small amount of harmful phosgene gas. This poisonous gas was used in the World War.
– r12 refrigerant properties
It is completely a safe refrigerant non-toxic, non-flammable, and non-explosive, highly stable compound under extreme operating conditions.
However, when brought in contact in an open flame or electric heating element R12 decomposes into products which are highly toxic.
R12 condenses at a moderate pressure under normal atmospheric temperature, has a boiling point of -29°C.
R12 is oil-miscible under all operating conditions, simplifying the problem of oil return thereby increasing the system efficiency.
The refrigerating effect per pound for R12 is comparatively low as compared to other refrigerants.
A Halide torch is used for leak detection.
R12 was completely phased out due to its ozone depleting potential (ODP) = 1 and Global Warming Potential (GWP) = 10000.
– r22 refrigerant properties
r22 has a boiling point of -40.7°C, developed primarily for low-temperature systems.
r22 was used extensively in domestic, commercial as well as industrial low-temperature systems to evaporator temperatures as low as -87°C.
Both atmospheric pressure and discharge temperature are higher compared to R12, but the power requirement is comparatively the same.
Evaporator temperatures are between -28 to -40°C.
The ability of r22 to absorb moisture is comparatively greater than r12, therefore, less trouble due to ice formation.
Fluorocarbon based refrigerants are safe.
Use a halide torch for leak detection.
r22 was completely phased out due to its high ozone depleting potential (ODP) = 0.05 and Global Warming Potential (GWP) = 1100.
How did some refrigerants | HCFCs and CFCs get banned?
The ozone layer in our atmosphere provides a filter for ultraviolet radiation, which can be harmful to our health.
Research has found that the ozone layer is thinning, due to emissions into the atmosphere by the substance like Chlorofluorocarbons (CFCs), HydroChloroFluoroCarbons (HCFC), halons and bromides.
Montreal and Kyoto international protocols and elimination of the chlorine-containing CFC and HCFCs in new equipment have successfully countered ozone depletion.
Some refrigerants used in the past are:
- Ammonia is highly explosive and toxic.
- Carbon dioxide requires high condensing pressure 72 bar at 30°C and the piping system is expensive.
- Methyl chloride is explosive and toxic
CFC and HCFC refrigerants (Freon, Arcton, etc.) and given R numbers (R12, R22, R502) are replaced by HFCs like R134a.
Due to the chlorine molecules’ ability to destroy the earth’s Ozone Layer by converting it to oxygen the international communities have agreed through a number of conventions to bring about its phase-out of such refrigerants.
- The Montreal protocol in 1985 agreed to reduce production and usage of CFC is a chlorofluorocarbon.
- The Montreal protocol in 1992 agreed to reduce production and usage of HCFC is HydroChloroFluoroCarbons.
- The European Union banned CFC’s in 1994 and HCFC.
The replacements of the CFC and HCFC refrigerants are the HFC’s.
r134a is an HFC refrigerant.
– r11 refrigerant (CCl3F) properties
R-11 is a fluorocarbon of the methane series and has a boiling point of 23.7°C at atmospheric pressure.
Like other fluorocarbon compounds, it dissolves natural rubber.
It is non-corrosive, non-toxic, non-flammable.
Use halide torch for leak detection.
R11 was completely phased out due to its high ozone depleting potential (ODP) = 1 and Global Warming Potential (GWP) = 3300.
CCl2 ⇒ CCl2F + Cl (Presence of U.V rays and sunlight)
Cl + O3 ⇒ ClO + O2 ( O3 is represented as Ozone)
ClO + O3 ⇒ Cl + 2O2 ( O2 is Oxygen)
Chlorine molecules have the ability to destroy earth’s Ozone Layer by converting it to oxygen.
One molecule of chlorine can destroy 1000 molecules of O3.
What are Environment-friendly Green Refrigerants?
- ODP (ozone depleting potential) of the refrigerant must be zero as it destroys the ozone layer leading to ultraviolet
- GWP (Global Warming Potential) of the refrigerant must be low as it may cause an increase in global warming.
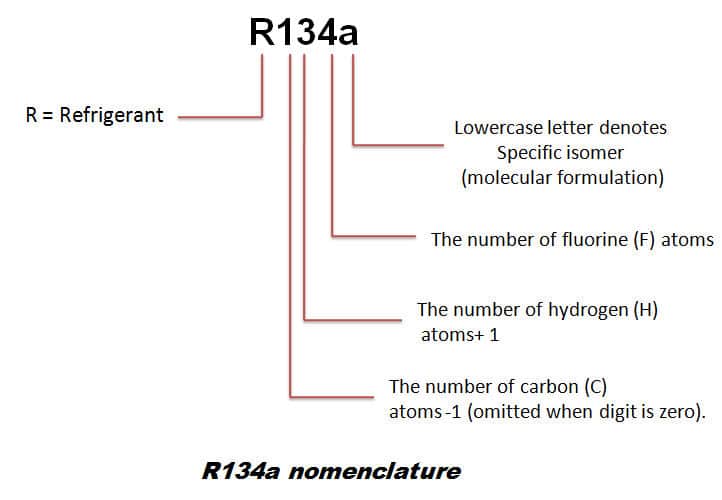
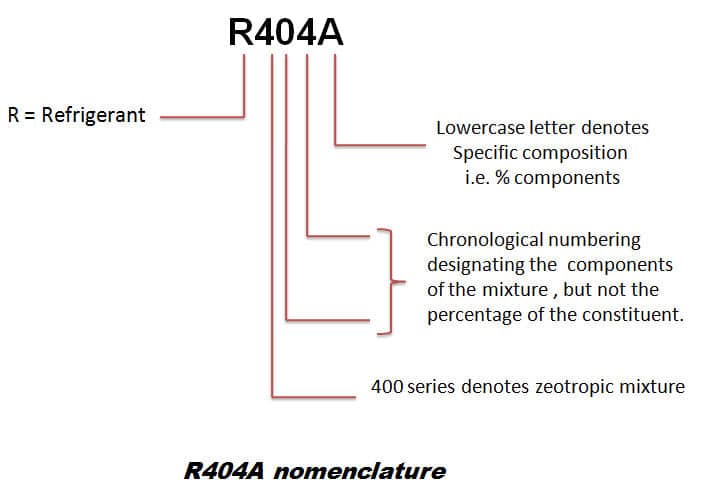
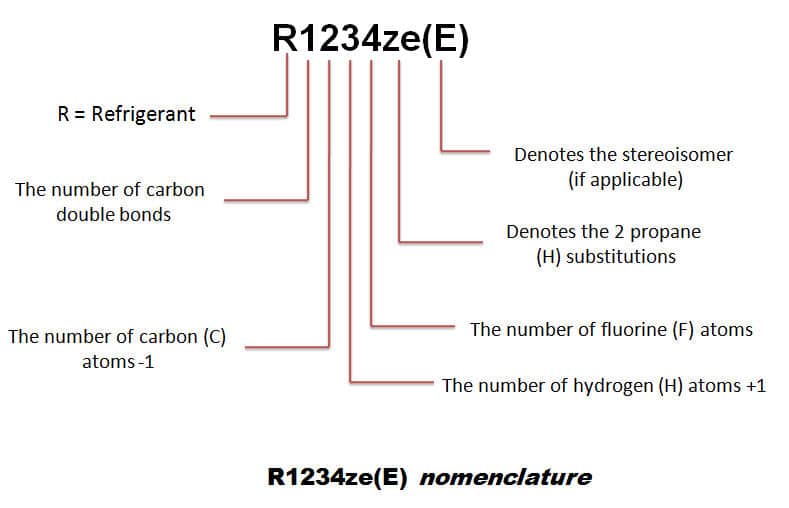
a) r134a refrigerant properties
R134a is capable to be at temperatures of -28°C at room temperatures of 38°C, its performance being very similar to that of an R12. However, the compressor must use a synthetic polyester lubricating oil.
Key properties of the main refrigerants are given in together with their typical application ranges;
| Low Temperature Refrigerant | −25℃ to –40°C |
| Medium Temperature Refrigerant | −5℃ to –25°C |
| High Temperature Refrigerant | +10 to –5°C |
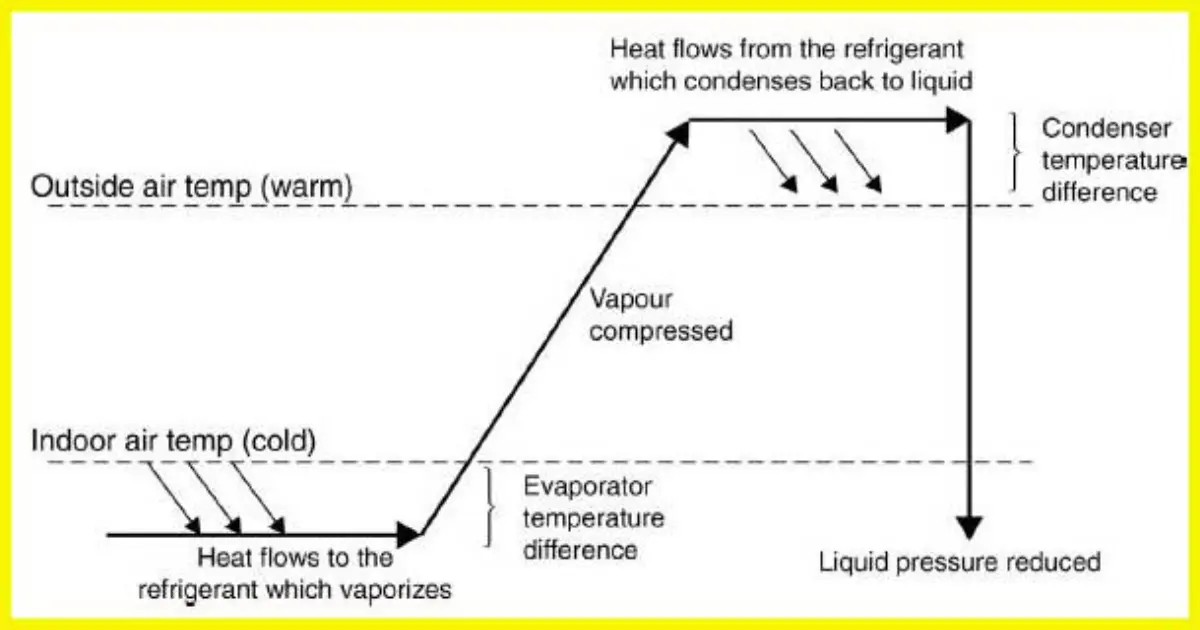
The key to a refrigeration system is to transfer heat energy from the evaporator to the condenser.
In order to achieve this, the compressor circulates refrigerant around the system which changes state as it receives and rejects heat.
b) r134a and r407c refrigerant properties
These refrigerants are primarily used for air conditioning and heat pumps and have replaced R22 in many applications. R134a has relatively low pressure and therefore about 50% larger compressor displacement is required when compared to R22.
The blended refrigerant R-134a is a long-term, it is an HFC alternative with similar properties to R-12.
R134a effectively works in screw chillers where short pipe lengths minimize costs associated with larger tubing. R134a also finds a position where high condensing temperatures are needed and in many transport applications
The HFO (hydro fluoro olefin) has zero ODP (ozone-depleting potential) and very low GWP (global warming potential) refrigerants R1234yf and R1234ze operate at similar pressures to R134a and are becoming available as long-term alternatives.
However, they are relatively high in cost and are flammable to a certain extent. R1234yf selected as a car air conditioning replacement.
R407C is a zeotropic mixture consisting of 23% R32, 25% R125 and 52% R134a. It has properties close to those of R22, and for this reason, has been extensively used in Europe.
Due to a rapid R22 phase-out. Its glide and heat transfer properties generally penalize the system performance, although counterflow heat exchange can deliver some benefit with plate type heat exchangers.
To find longer-term alternatives, it is necessary to move to R32 HFO blends or R717, all of which require significant system re-design.
c) r410a refrigerant properties
R410a is a high-pressure refrigerant with a low critical temperature used mainly in ac compressor. With proper system design, it has been shown to give equivalent or better performance than R407C.
Many air conditioning suppliers switched to R410a from R22, especially for direct expansion-type systems where an added advantage is the use of smaller pipe sizes.
R32 is a possible longer-term alternative. It is already a 50% component of R410A, but alone it is flammable to a limited extent.
d) r404a refrigerant properties
R404A is an HFC blend designed excessively for commercial refrigeration. It has superior performance against many other HFCs in low-temperature applications.
It also exhibits low compressor discharge temperatures which makes it suitable for single-stage compression, avoiding the need for inter-stage cooling.
Its high GWP makes it unsuitable for future use, and it’s close to a rapid phase-out. Medium-term replacements are R407A and R407F.
HFO/HFC blends an alternative for the future. A large proportion of users are investing in R744 technology as a long-term solution.
R404a Ozone depleting potential = 0 and Global Warming Potential = 3260.
e) r717 ammonia properties
Ammonia is still, without a doubt, the most important industrial refrigerant in the present day because of its good thermodynamic properties and it’s cheap.
Ammonia is one of the refrigerants most commonly used in the absorption-type air-conditioning systems. Ammonia vapors are absorbed quickly by large amounts of cool water. In fact, it can absorb vapor as quickly as a compressor.
Due to its high toxicity and flammability, an industrial application using ammonia requires strict regulations.
The extent of technical developments for ammonia are increasing for e.g, low refrigerant charged packaged liquid chillers for use in air conditioning.
Ammonia is not compatible with copper and its alloys, so refrigerant piping and components have to be made of steel or aluminum.
Ammonia has a lesser density compared to air, so in case of any leakage, ammonia mixes into the atmosphere.
If the industrial plant is built outside or on the roof of a building, the escaping ammonia can easily drift away without harming the occupants.
Ammonia can be detected by its odor at very low concentrations, and this acts as an early warning signal.
The safety aspects of ammonia plants are well known, and there is a reason to expect a continued increase in the use of ammonia as long as refrigerant exists.
Ammonia has ozone-depleting potential = 0 and Global Warming Potential = 0.
f) r-401b Refrigerant properties
This blend refrigerant is similar to R-401A except, it is higher in R-22 content. This blend has a higher capacity at lower temperatures and matches R-12 at –20°F. It also provides a closer match to R-500 at air-conditioning temperatures.
Applications for R-401B are in normally lower temperature R-12 refrigeration locations and in transport refrigeration, and in R-500 as a direct expansion refrigerant in air-conditioning systems.
g) Carbon Dioxide refrigerant properties
Carbon dioxide is a colorless gas without any definite smell at ordinary temperature.
It requires a high condensing pressure of 72 bar at 30°C to liquefy.
It requires an expensive piping system to handle high condensing pressure.
Carbon dioxide is hazardous to human life with a concentration above 5 %. It is thermally stable and does not decompose until the temperature is well over 1000°C.
A CO2 concentration greater than 2% causes putrefaction of some fruits especially apples and pears into an internal brown color core.
Core putrefaction is due to anaerobic bacteria.

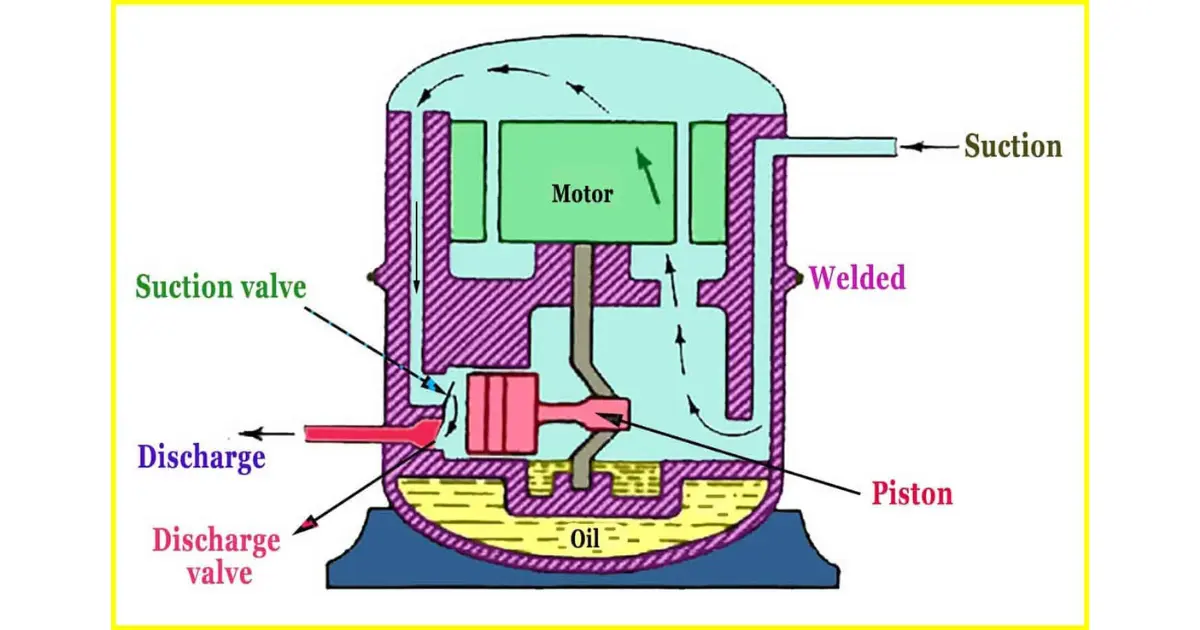
is refrigerant gas R410A compatible with R404A